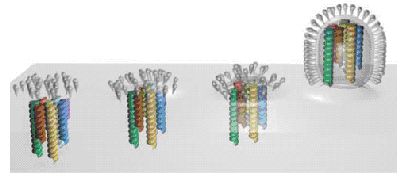
Assembly and
budding of virus particles is a complex process, which is so far not
well understood. Not only the viral proteins have to be segregated
selectively into the budding site, also the genetic material has to be
packet into the virus core (Fig. 1).
The Influenza virus buds from the apical plasma membrane of epithelial
cells where the viral components are assembled in a well organised
manner. The envelope proteins of Influenza A are known to be
specifically targeted to the budding site, but very little is known
about how the core proteins enclosing the viral genome are targeted and
incorporated into the progeny virus particles.
The genome
of each influenza virus particle consists of eight different
single-stranded negative-sense RNA segments, each of which is associated
with nucleoprotein and three polymerase subunits (PA, PB1 and PB2) (Fig.
2). They are assembled in the nucleus and then transported to the
budding site at the plasma membrane.
In this project we focus on the interactions of the eight
ribonucleoprotein (RNP) complexes containing the negative strand genomic
RNA of Influenza with lipids and proteins that might contribute to the
assembly of the eight segments into the budding virions.