Fluorine Chemistry, Organometallic Chemistry, Homogeneous and Heterogeneous Catalysis
The research of the Braun group is interdisciplinary with a focus on the development of novel reaction routes in homogeneous catalysis. The chemical synthesis of model compounds and mechanistic studies contributing to the understanding of catalytic reactions are of fundamental importance. This requires the use of various analytical tools like NMR, IR and Raman spectroscopy, mass spectrometry and single crystal X-ray diffraction. In an additional spin-off is dedicated to heterogeneous catalysis and C-H as well as C-F activation reactions at aluminium fluorides. This includes the identification of surface species as well as model reactions on a molecular level. With a comparable intention to unravel surface chemistry, model reactions for hydrolysis and flurorination reactions at molecular Al and Si compounds are currently developed.
This research is combined with a deep interest in fluorine chemistry. Thus, the Braun group performs research in inorganic and organic fluorine chemistry. This involves again the development of new catalytic reactions, of new fluorinated ligands, but also of new fluorinating reagents. A special focus is on sulfur fluorine chemistry. This imparts the activation of the greenhouse gas SF6, but also the development of new reactivity pathways of reagents such as SF4, SF5Br or SF5Cl. Of high interest is also the development of reactions to convert green-house gases into fluorinated building blocks, which might useful for materials and pharmaceuticals.
1. Transition metal-mediated C–F and C-H bond Activation and Derivatization of Fluorinated Compounds
2. Chemistry of Sulfur Fluorides
3. Reactive Transition Metal Fluorido Complexes as Fluorinating Agents
5. Rhodium and Iridium Peroxido Complexes as Oxygenation Agents
6. Si and Al Compounds as Molecular Models for Surface Reactions
1. Transition metal-mediated C–F and C-H bond Activation and Derivatization of Fluorinated Compounds
Fluorinated materials and compounds are of an enormous importance in material science as well as for pharmaceuticals and agrochemicals. Studies of the Braun group are concerned with the development of new reaction routes for the synthesis of fluorinated compounds. The strategies involve catalytic C-H and C-F activation reactions, fluorination reactions as well as the development of new fluorinating agents. The replacement of a fluorine atom in a highly fluorinated molecule (i.e. a C-F activation) is a unique strategy to access new fluorinated building blocks.
The approach of the Braun group involves transition-metal mediated cleavage reactions and subsequent derivatizations of the metalated fluorinated moieties. Typical examples involve conversions of fluorinated aromatics, heteroaromatics and olefins. Mechanistic studies are of considerable importance and include low temperature NMR, IR and Raman investigations as well as DFT calculations. Based on stoichiometric reactions, usually catalytic processes are developed to access new fluorinated compounds and materials. Two examples for C-F activation reactions and catalytic transformations are shown below.
Treatment of the hydrido complex [RhH(PEt3)3] with hexafluoropropene gives the C-F activation product [Rh{(Z)-CF=CF(CF3)}(PEt3)3]. The latter is a good catalyst for the synthesis of silylated and borylated fluoroalkanes.
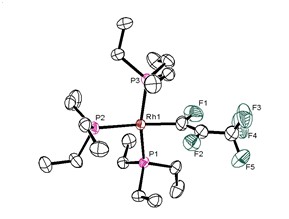
Another example shows that the highly reactive rhodium(I) complex [Rh(Bpin)(PEt3)3] (pin = pinacolato) is able to activate C−F bonds, but also C–H bonds of fluorinated molecules. As a result, borylated heterocyclic molecules were obtained via such derivatization reactions, and the fluorinated building blocks are not accessible otherwise. DFT calculation revealed a unique C-F bond cleavage step which is boryl-assisted. Rhodium germyl and silyl complexes were employed in a comparable way, and for the latter even refluorination reactions were found. The rhodium germyl complex [Rh(GePh3)(PEt3)3] is in addition able to catalyze hydrogermylation reactions at fluorinated alkenes.
Unique catalytic C-H borylation reactions of SCF3 containing compounds were also developed. The borylation steps occur at the ortho position of the SCF3 group and it is believed that the latter is ortho directing.
Selected literature:
C. N. von Hahnmann, M. Talavera, C. Xu, T. Braun, Chem. Eur. J. 2018, 24, 11131-11138.
„Reactivity of 3,3,3-Trifluoropropyne at Rhodium Complexes: Development of Hydroboration Reactions“
T. Ahrens, M. Teltewskoi, M. Ahrens, T. Braun, R. Laubenstein, Dalton Trans. 2016, 45, 17495-17507.
„Competing reaction pathways of 3,3,3-trifluoropropene at rhodium hydrido, silyl and germyl complexes: C-F bond activation versus hydrogermylation“
T. Ahrens, T. Braun, B. Braun, R. Herrmann, Dalton Trans. 2016, 45, 4716-4728.
„Synthesis of a rhodium(I) germyl complex: a useful tool for C-H and C-F bond activation reactions“
S. I. Kalläne, M. Teltewskoi, T. Braun, B. Braun, Organometallics 2015, 34, 1156-1169.
„C-H and C-F Bond Activations at a Rhodium(I) Boryl Complex: Reaction Steps for the Catalytic Borylation of Fluorinated Aromatics“
A. L. Raza, T. Braun, Chem. Sci. 2015, 6, 4255-4260.
„Consecutive C-F bond activation and C-F bond formation of heteroaromatics at rhodium: the peculiar role of FSi(OEt)3„
S. I. Kalläne, T. Braun, M. Teltewskoi, B. Braun, R. Herrmann, R. Laubenstein, Chem. Commun. 2015, 51, 14613-14616.
„Remarkable reactivity of a rhodium(I) boryl complex towards CO2 and CS2: isolation of a carbido complex“
S. I. Kalläne, T. Braun, Angew. Chem. 2014, 126, 9465-9469; Angew. Chem. Int. Ed. 2014, 53, 9311-9315.
„Catalytic Borylation of SCF3-Functionalized Arenes by Rhodium(I) Boryl Complexes: Regioselective C-H Activation at the ortho-Position“
A. L. Raza, J. Panetier, M. Teltewskoi, S. Macgregor, T. Braun, Organometallics 2013, 32, 3795-3807.
„Rhodium(I) Silyl Complexes for C–F Bond Activation Reactions of Aromatic Compounds: Experimental and Computational Studies“
M. Teltewskoi, J. A. Panetier, Stuart A. Macgregor, T. Braun, Angew. Chem. 2010, 122, 4039-4043; Angew. Chem. Int. Ed. 2010, 49, 3947-3951.
„A Highly Reactive Rhodium(I)-Boryl Complex as a Useful Tool for C-H Bond Activation and Catalytic C-F Bond Borylation„
2. Chemistry of Sulfur Fluorides
The pentafluorosulfanyl group is characterized by its very high electronegativity and its significant steric demand. Due to their increased lipophilicity, organopentafluorosulfanyl compounds are regarded as promising building blocks in pharmacy and agrochemistry. To access SF5 containing aromatic building blocks bromo and iodo aromatics were converted into borylated pentafluorosulfanyl compounds by treatment with B2pin2 (pin = pinacolato) on employing the catalyst precursor [Pd(Me)2(tmeda)] (tmeda = N,N,N‘,N‘-tetramethylethylenediamine) in the presence of free phosphine and CsF as a base. Mechanistic studies revealed the crucial role of intermediate palladium fluorido complexes to induce the borylations. A similar importance of fluoride complex was recently discovered, when mechanistic studies on cross coupling reactions were performed to provide a new route to fluorinated amino acids.
Inert S–F bonds in SF5-moieties and even SF6 can be activated at the binuclear rhodium hydrido complex [{Rh(μ-H)(dippp)}2] (dippp=1,3-bis(diisopropylphosphanyl)propane) resulting in a complete defluorination. In case of the pentafluorosulfanyl compounds an additional cleavage of the C–S bond takes place in the presence of HSiEt3, to afford [Rh2(μ-H)(μ-SSiEt3)(dippp)2], FSiEt3, and H2. Subsequent studies revealed that a selective catalytic depletion of SF6 on employing [Rh(H)(PEt3)3] in the presence of free phosphine and HSiEt3 is possible, to convert SF6 into fluorophosphoranes and fluorosilanes as non-volatile products. Further developments revealed that SF6, which exhibits a very high global warming potential, can even be converted into platinum bound SF3 ligands, an subsequently used as fluorinating agent. Reactivity studies revealed that the PtSF3 compounds can be used to fluorinate alcohols or ketones.
It was also known that N-heterocyclic carbenes can mediate fluorination reactions on using the inert greenhouse gas SF6 as fluorinating agent. The transformation are initiated photochemically to produce fluorinated derivatives of the carbenes, which than can be used for the fluorination of alcohols. A one-pot process was also developed.
Selected literature:
L. Zámostná, T. Braun, B. Braun, Angew. Chem. 2014, 126, 2783-2787; Angew. Chem. Int. Ed. 2014, 53, 2745-2749.
„S-F and S-C Activation of SF6 and SF5 Derivatives at Rhodium: Conversion of SF6 into H2S“
L. Zámostná, T. Braun, Angew. Chem. 2015, 127, 10798-10802; Angew. Chem. Int. Ed. 2015, 54, 10652-10656.
„Catalytic Degradation of Sulfur Hexafluoride by Rhodium Complexes“
C. Berg, R. Laubenstein, T. Braun, B. Braun, Chem. Commun. 2016, 52, 3931-3934.
„Palladium-mediated borylation of pentafluorosulfanyl functionalized compounds: the crucial role of metal fluorido complexes“
C. Berg, T. Braun, M. Ahrens, P. Wittwer, R. Herrmann, Angew. Chem. 2017, 129, 4364-4368; Angew. Chem. Int. Ed. 2017, 56, 4300-4304.
„Activation of SF6 at Platinum Complexes: Formation of SF3 Derivatives and Their Application in Deoxyfluorination Reactions“
M. Wozniak, T. Braun, M. Ahrens, B. Braun-Cula, P. Wittwer, R. Herrmann, R. Laubenstein, Organometallics 2018, 37, 821-828.
„Activation of SF6 at a Xantphos-Type Rhodium Complex“
C. Berg, N. Pfister, T. Braun, B. Braun-Cula, Chem. Eur. J. 2018, 24, 7985-7990.
„Diverse Reactivity of Platinum SF3 and SF2 Complexes towards EtOH and Me3SiOEt“
P. Tomar, T. Braun, E. Kemnitz, Chem. Commun. 2018, 54, 9753-9756.
„Photochemical activation of SF6 by N-heterocyclic carbenes to provide a deoxyfluorinating reagent“
N. Pfister, T. Braun, P. Wittwer, M. Ahrens, Z. Anorg. Allg. Chem. 2018, 644, 1064-1070.
„Selective Formation and Characterization of a RhIII λ4-Trifluorosulfanyl Complex„
3. Reactive Transition Metal Fluorido Complexes as Fluorinating Agents
Metal fluorido complexes often exhibit very unusual properties due to the small size and the high electronegativity of the fluorine atom. On treatment of palladium, platinum, rhodium or iridium complexes with the mild HF-source Et3N · 3HF various compounds bearing fluorido ligands are accessible which were studied towards their ability to act as fluorinating agents.
For instance, platinum(0) alkyne complexes that are stabilized by chelating ligands are capable to react selectively with the electrophilic fluorinating agent NFSI (N-fluorobenzenesulfonimide). Depending on the reaction conditions, a monofluorination of the alkyne ligand or the metal center is achieved. The monofluorinated alkene can be liberated by reaction with hydrogen.
Recent projects involve the generation of N-heterocyclic carbine complexes of gold to use them as catalysts for fluorination. This includes the generation of Au(I) compounds. In cooperation with the research group of Sebastian Hasenstab-Riedel at the Freie Universität Berlin Au(III) fluorido complexes unique were synthesized, which can be used as fluorinating agents.
Selected literature:
P. Kläring, T. Braun, Angew. Chem. 2013, 125, 11302-11307; Angew. Chem. Int. Ed. 2013, 52, 11096-11101.
„Insertion of CS2 into Iridium–Fluorine Bonds“
J. Berger, T. Braun, R. Herrmann, B. Braun, Dalton Trans. 2015, 44, 19553-19565.
„ Reactivity of platinum alkyne complexes towards N-fluorobenzenesulfonimide: formation of platinum compounds bearing a β-fluorovinyl ligand“
M. A. Ellwanger, S. Steinhauer, P. Golz, H. Beckers, A. Wiesner, B. Braun-Cula, T. Braun, S. Riedel, Chem. Eur. J. 2017, 23, 13501-12509.
„Taming the High Reactivity of Gold(III) Fluoride: Fluorido Gold(III) Complexes with N-Based Ligands“
J. Berger, T. Braun, T. Ahrens, P. Kläring, R. Laubenstein, B. Braun-Cula, Chem. Eur. J. 2017, 23, 8886-8900.
„The Versatile Behavior of Platinum Alkyne Complexes towards XeF2: Formation of Fluorovinyl and Fluorido Complexes“
M. A. Ellwanger, S. Steinhauer, P. Golz, T. Braun, S. Riedel, Angew. Chem. 2018, 130, 7328-7332; Angew. Chem. Int. Ed. 2018, 57, 7210-7214.
„Stabilization of Lewis Acidic AuF3 as an N-Heterocyclic Carbene Complex: Preparation and Characterization of [AuF3(SIMes)]“
M. A. Ellwanger, C. von Randow, S. Steinhauer, Y. Zhou, A. Wiesner, H. Beckers, T. Braun, S. Riedel, Chem. Commun. 2018, 54, 9301-9304.
„Tuning the Lewis acidity of difluorido gold(III) complexes: the synthesis of [AuClF2(SIMes)] and [AuF2(OTeF5)(SIMes)]“
4. Heterogeneously Catalyzed C–F, C–Cl and C–H Bond Activation Reactions at Lewis-acidic Aluminium Fluorides
Solid Lewis acids are the basis of numerous heterogeneously catalyzed reactions and processes. The nanoscopic compound aluminum chlorofluoride (ACF, AlClxF3-x, x = 0.05-03; DuPont, US 5 157 171, 1992) exhibits a Lewis acidity strength comparable to SbF5 and can be applied for heterogeneously catalyzed reactions. The developed conversions involve C-H activation reactions of methane and other alkanes, hydroarylation reactions of olefins as well as various C-F activation reactions of fluorinated alkanes. Thus by reaction with Et3SiH with ACF, one generates silylium-like species of the type ACF…H…SiEt3 at the ACF surface. These species were successfully applied in C–X bond activation reactions. Depending on the reaction conditions, chloro- and fluoromethanes were converted into methane via hydrodehalogenation reactions or into the corresponding Friedel-Crafts products.
It is intriguing that reactions of fluoroalkanes such fluoroheptane result at ACF…H…GeEt3 in dehydrofluorination reactions yielding olefins, whereas at ACF…H…SiEt3 hydrodefluorination and Friedel-Crafts type reactions were observed. Furthermore, selective defluorinations and germylations of the refrigerant 2,3,3,3-tetrafluoropropene (HFO-1234yf), which is used in automobile air conditioners, as well as defluorination reactions of 2-chloro-1,1,1,2-tetrafluoropropane (HCFC-244bb) to give 2-chloro-3,3,3-trifluoropropene (HFO-1233xf) were developed. The latter transformations are of importance for the development of new reaction routes for a derivatization of fluorinated compound which have a high global warming and/or ozone depletion potential. Mechanistic studies involve the use of MAS-NMR and vibrational spectroscopic methods, as well as DTA, TG and TPD measurements.
Recently, several new mesoporous catalysts were developed which are able to catalyse a variety of different catalytic reactions, such as the cyclization of citronellal to isopulegol. These materials conists of Nb-doped variants of a high surface aluminium fluoride.
Selected literature:
M. Ahrens, G. Scholz, T. Braun, E. Kemnitz, Angew. Chem. 2013, 125, 5436-5440; Angew. Chem. Int. Ed. 2013, 52, 5328-5332.
„Catalytic Hydrodefluorination of Fluoromethanes at Room Temperature by Silylium-ion-like Surface Species“
A. K. Siwek, M. Ahrens, M. Feist, T. Braun, E. Kemnitz, ChemCatChem 2017, 9, 839-845.
„Activation of Chlorinated Methanes at the Surface of Nanoscopic Lewis Acidic Aluminum Fluorides“
G. Meißner, D. Dirican, C. Jäger, T. Braun, E. Kemnitz, Catal. Sci. Technol. 2017, 7, 3348-3354.
„Et3GeH versus Et3SiH: controlling reaction pathways in catalytic C-F bond activations at a nanoscopic aluminium chlorofluoride“
G. Meißner, M. Feist, T. Braun, E. Kemnitz, J. Organomet. Chem. 2017, 847, 234-241.
„Selecive reduction of a C-Cl bond in halomethanes with Et3GeH at nanoscopic Lewis acidic Aluminium fluoride Formation of Fluorovinyl and Fluorido Complexes“
G. Meißner, K. Kretschmar, T. Braun, E. Kemnitz, Angew. Chem. 2017, 129, 16556-16559; Angew. Chem. Int. Ed. 2017, 56, 16338-16341.
„Consecutive Transformations of Tetrafluoropropenes: Hydrogermylation and Catalytic C−F Activation Steps at a Lewis Acidic Aluminum Fluoride“
B. Calvo, T. Braun, E. Kemnitz, ChemCatChem 2018, 10, 403-406.
„Hydrogen/Deuterium-Exchange Reactions of Methane with Aromatics and Cyclohexane Catalyzed by a Nanoscopic Aluminium Chlorofluoride“
C. P. Marshall, T. Braun, E. Kemnitz, Catal. Sci. Technol. 2018, 8, 3151-3159.
„Modifying the reactivity of a solid Lewis acid: niobium and antimony doped nanoscopic aluminum fluoride“
B. Calvo, C. P. Marshall, T. Krahl, J. Kröhnert, A. Trunschke, G. Scholz, T. Braun, E. Kemnitz, Dalton Trans. 2018, 47, 16461-16473.
„Comparative study of the strongest Lewis acids known: ACF and HS-AlF3„
M. Kervarec, C. P. Marshall, T. Braun, E. Kemnitz, J. Fluorine Chem. 2019, 221, 61-65.
„Selective dehydrofluorination of 2-chloro-1,1,1,2-tetrafluoropropane (HCFC-244bb) to 2-chloro-3,3,3-trifluoropropene (HFO-1233xf) using nanoscopic aluminium fluoride catalysts at mild conditions“
5. Rhodium and Iridium Peroxido Complexes as Oxygenation Agents
Another topic consists of the stabilization of unusual peroxido units in the coordination sphere of transition metals. Numerous iridium and rhodium peroxido species were prepared starting from oxygen. The conversions include reactions with 3O2, in situ-generated 1O2 and redox-mediated oxygenations. Reactivity studies led to oxygenation reactions at nitriles and olefins as well as to the formation of H2O2 from O2 and H2.
Selected literature:
H. Baumgarth, G. Meier, C. N. von Hahmann, T. Braun, Dalton Trans. 2018, 47, 16299-16304.
„Reactivity of rhodium and iridium peroxido complexes towards hydrogen in the presence of B(C6F5)3 or [H(OEt2)2][B{3,5-(CF3)2C6H3}4]„
H. Baumgarth, G. Meier, T. Braun, B. Braun-Cula, Eur. J. Inorg. Chem. 2016, 4565-4572.
„Rhodium and Iridium Fluorido and Bifluorido Complexes Derived from Peroxido Precursors“
A. Bittner, T. Braun, R. Herrmann, S. Mebs, Chem. Eur. J. 2015, 21, 12299-12302.
„Rhodium-Mediated Oxygenation of Nitriles with Dioxygen: Isolation of Rhodium Derivatives of Peroxyimidic Acids“
G. Meier, T. Braun, Angew. Chem. 2012, 124, 12732-12737; Angew. Chem. Int. Ed. 2012, 51, 12564-12569.
„Hydrogenation of a Rhodium Peroxido Complex by Formate Derivatives: Mechanistic Studies and the Catalytic Formation of H2O2 from O2„
G. Meier, T. Braun, Angew. Chem.2011, 123, 3338-3342; Angew. Chem. Int. Ed. 2011, 50, 3280-3284.
„A Rhodium Peroxide Complex in Mono-, Di- and Peroxygenation Reactions“
6. Si and Al Compounds as Molecular Models for Surface Reactions
Zeolites are produced in large scale via solvothermal methods, but the understanding of the corresponding hydrolysis and condensation steps are still the subject of current research. Another aspect consists of a structural modeling of the reactive surface sites on a molecular level. This includes a modeling of conceivable surface defect structures. Subvalent aluminum and silicon compounds like AlCp* oder (Mes)2Si=Si(Mes)2 (Cp* = C5Me5; Mes = 2,4,6-Trimethylphenyl) were used to investigate oxygenation and subsequent hydrolysis and condensation reactions.
It was for instance shown that the oxygenation of tetrameric [AlCp*]4 yields the heterocubic compound [Cp*AlO]4 which in turn can be converted by controlled hydrolysis into two different molecular entities representing structural motifs included in the solid state structures of boehmite and diaspore. Investigations on the reactivity of Cp*Al towards Silanols led in the presence of water to hydrolysis of Alumosiloxanes a unique [Al7(OH)9(O3Si2iPr4)6] Cluster.
Several adducts of Mes2Si(OH)(µ-O)Si(OH)Mes2 with ether molecules were characterised in the solid state by X-ray crystallography and ATR IR spectroscopy. The compounds were obtained by crystallisation from mixtures in C6D6 and various ethers such as Et2O, dme or dioxane and are only stable in the solid state. They form polymeric chain-like structures and the Mes2Si(OH)(µ-O)Si(OH)Mes2 moieties are linked by ether molecules via hydrogen bonding bridges. Moreover a silylene-borane lewis-pair was used as a tool for trapping a single water molecule. This led to silanol formation and dehydrogenation reactions.
Selected literature:
P. Roesch, R. Müller, A. Dallmann, G. Scholz, M. Kaupp, T. Braun, B. Braun-Cula, P. Wittwer, Chem. Eur. J. 2019, 25, 4678-4682.
„A Silylene-Boran Lewis Pair as a Tool for Trapping Water Molecule: Silanol Formation and Dehydrogenation“
P. Wittwer, A. Stelzer, T. Braun, Eur. J. Inorg. Chem. 2018, 27, 3187-3194.
„Reactivity of Cp*Al towards Silanols: Formation and Hydrolysis of Alumosiloxanes“
P. Roesch, U. Warzok, M. Enke, R. Müller, C. Schattenberg, C. A. Schalley, M. Kaupp, T. Braun, P. Wittwer, Chem. Eur. J. 2017, 23, 13964-13972.
„Reactivity of the Sterically Demanding Siloxanediol Mes2Si(OH)-(μ-O)Si(OH)Mes2 Towards Water and Ether Molecules“
A. C. Stelzer, P. Hrobárik, T. Braun, M. Kaupp, B. Braun-Cula, Inorg. Chem. 2016, 55, 4915-4923.
„Completing the Heterocubane Family [Cp*AlE]4 (E = O, S, Se, and Te) by Selective Oxygenation and Sulfuration of [Cp*Al]4: Density Functional Theory Calculations of [Cp*AlE]4 and Reactivity of [Cp*AlO]4 toward Hydrolysis“